Background: Autologous CAR-T therapy targeting CD19 is an effective treatment for relapsed or refractory large B-cell lymphoma (LBCL). However, morbidity and mortality related to CAR-T toxicity remain significant concerns. This study aims to evaluate the patterns, risk factors, and implications of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) following CD19-CAR-T therapy in LBCL.
Methods: This retrospective analysis includes 1916 LBCL patients treated with CD19-CAR-T cell therapy (axicabtagene-ciloleucel [axi-cel] 75% and tisagenlecleucel 25%) between 2018 and 2020 and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). Patient demographics, baseline characteristics, treatment-related variables, and clinical outcomes were collected. The incidence, severity and timing of onset of CRS and ICANS (according to median time to onset) were analyzed, factors associated with these outcomes and their impact on overall survival as time dependent covariates were done using Cox Regression multivariate analyses.
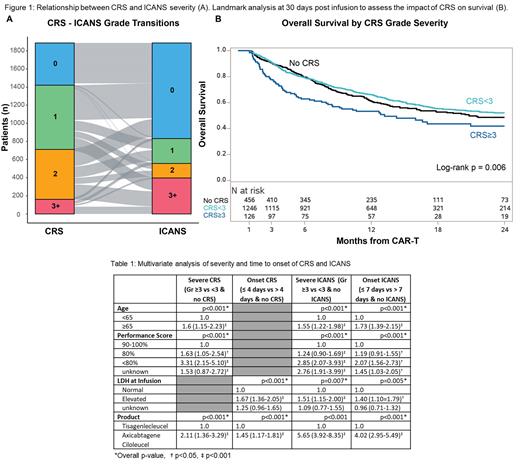
Results: The median age of the patients was 64 years, with a majority having a Karnofsky performance status (KPS) below 90% (61%) and not receiving bridging therapy (67%). CRS was observed in 75.1% of patients, with 9% experiencing severe CRS (grades ≥ 3). Similarly, 43% of patients experienced ICANS, with 20% having severe ICANS (grade ≥ 3). The median time to toxicity was shorter for CRS (4 days) compared to ICANS (7 days). Nearly all the patients who developed ICANS also experienced CRS (98%; ), and the correlation between these two events and respective severities are shown in the Figure 1a. In the multivariate analyses, factors associated with development of CRS were gender (women, Odds ratio [OR] 1.39, 95% confidence interval [CI] 1.10- 1.76, p=0.006), high LDH at time of infusion (OR 1.56; 95% CI 1.22 - 2.00, p=0.002) and product (axi-cel OR 4.60, 95% CI 3.56 -5.81, p<0.001). Factors associated with the development of ICANS included age (> 65 years, OR 1.83; 1.50-2.24, p<0.001), KPS (<80%, OR 2.48; 1.88-3.27, p<0.001), disease status at infusion (treatment resistant disease, OR 1.98; 1.09-3.58, p=0.024; and untreated relapse OR 2.62, 1.35-5.10, p=0.004) and product (axi-cel, OR 5.85; 4.47-7.64, p<0.0001). Factors associated with severe and early onset CRS and ICANS are listed in the table below.
The median follow-up of 14.2 months, the estimated 12-month overall survival, progression-free survival (PFS), and treatment-related mortality were 61.6% (95% CI: 59.4-63.9), 42.2% (39.9-44.5), and 4.3% (3.4-5.3), respectively. Overall CRS, early onset CRS and overall ICANS were not associated with higher mortality, however grade >3 CRS (HR 1.79; 1.41-2.28, p<0.001), grade >3 ICANS (hazard ratio [HR] 1.34; 95% CI 1.13-1.59, p=0.002) and early onset ICANS (<7 days, HR 1.23; 1.05-1.44, p=0.01) were associated with higher mortality. The impact of severe CRS on survival are shown in the landmark analysis at 30 days from infusion in the figure 1B.
Conclusion: In the largest analysis of CD19-CAR-T cell therapy toxicities to date, we observed a high burden of CRS and ICANS among LBCL patients. The CAR T cell product type was consistently associated with higher incidence, shorter onset and higher severity of both toxicities. High LDH, older age and low performance score also influence the severity and onset of these outcomes. Additionally, patients who developed higher grades of CRS/ICANS and earlier onset ICANS had a higher mortality, stressing the need to develop novel strategies to mitigate the incidence and optimize management of these patients to improve their outcomes.
Disclosures
Ahmed:ADC Therapeutics: Consultancy; Kite/Gilead: Consultancy; Servier: Consultancy; Chimagen: Research Funding; Genmab: Research Funding; Merck: Research Funding; Seagen: Research Funding; Tessa Therapeutics: Research Funding. Awan:Pharmacyclics LLC, an AbbVie Company.: Other: Contracted Research; Janssen, Gilead, Kite pharmaceuticals, Karyopharm, MEI Pharma, Verastem, Incyte, Johnson and Johnson, Merck, Epizyme, Loxo Oncology, Adaptive Biotechnologies, Genmab: Other: Consulting Agreements; AstraZeneca Pharmaceuticals LP: Other: Advisory Committee; AbbVie Inc, ADC Therapeutics, AstraZeneca Pharmaceuticals LP, BeiGene Ltd, Bristol-Myers Squibb Company, Cardinal Health, Caribou Biosciences Inc, Celgene Corporation, Cellectar Biosciences Inc, DAVA Oncology, Epizyme Inc, Genentech, a member of the Roche: Other: Consulting Agreements. Bachanova:ADC: Membership on an entity's Board of Directors or advisory committees; Citius: Research Funding; BMS: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Gamida Cell: Research Funding; Miltenyi: Other: DSMB. Barba:Pierre-Fabre: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Beitinjaneh:Kite: Honoraria. Cashen:Kite Pharma: Consultancy. Dholaria:Adicet: Research Funding; MEI: Research Funding; Orca Bio: Research Funding; Poseida: Research Funding; Allovir: Research Funding; NCI: Research Funding; Atara: Research Funding; Molecular Templates: Research Funding; AstraZeneca: Research Funding; Pluri Biotech: Consultancy; Angiocrine: Research Funding; Boxer Capital: Consultancy; Ellipsis pharma: Consultancy; Lumanity: Consultancy; Takeda: Research Funding; Arivan: Consultancy; ADC therapeutics: Consultancy, Honoraria; Gilead: Research Funding; Wugen: Research Funding; Pfizer: Research Funding; BEAM therapeutics: Consultancy; gamida cel: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Poseida: Research Funding. Elsawy:BMS/Celgene, Kite/Gilead, Pfizer, Abbvie, Janssen, Jazz Pharma, Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hashmi:Jannsen: Honoraria, Speakers Bureau; BMS: Honoraria; Karyopharm: Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau. Hill:March Biosciences: Consultancy; Kite/Gilead: Speakers Bureau. Jain:Loxo@Lilly: Research Funding; Myeloid Therapeutics: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Incyte: Research Funding. Jain:Care Dx, Bristol Myers Squibb, Incyte, Abbvie, CTI, Kite, Cogent Biosciences, Blueprint Medicine, Telios pharma, Protagonist therapeutics: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma, Kartos therapeutics, Incyte: Research Funding. Kebriaei:Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Kittai:BeiGene: Consultancy, Research Funding, Speakers Bureau; Eli Lilly: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbive: Consultancy; Janssen: Consultancy; KITE: Consultancy; BMS: Consultancy. Locke:ASH: Other: Travel Support; Leukemia and Lymphoma Society: Other; GammaDelta Therapeutics: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Clinical Care Options Oncology: Other; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy; Imedex: Other; CERo Therapeutics: Other: (Institutional); National Cancer Institute: Other; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Other: Travel Support; Emerging Therapy Solutions: Consultancy, Other; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Pfizer: Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Institutional; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Cowen: Consultancy; Cellular Medicine Group: Consultancy; Caribou: Consultancy; Calibr: Consultancy; Society for Immunotherapy of Cancer: Other; EcoR1: Consultancy; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Lulla:Bristol Myers Squibb, Marker Therapeutics and Allovir: Research Funding; Marker Therapeutics: Research Funding; Allovir: Research Funding. McGuirk:Novartis: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy; Allovir: Consultancy, Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Fresenius Biotech: Research Funding; Astellas Pharma: Research Funding; Bellicum Pharmaceuticals: Research Funding; Pluristem Therapeutics: Research Funding; Gamida Cell: Research Funding. Mussetti:Gilead: Research Funding; Takeda: Honoraria; BMS, Jazz Pharaceuticals: Consultancy. Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Perales:Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; Vor Biopharma: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Adicet: Honoraria; Syncopation: Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Kite: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Servier: Other; Exevir: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Sellas Life Sciences: Consultancy; Medigene: Consultancy, Other; Cidara Therapeutics: Consultancy, Other; Astellas: Consultancy, Honoraria; Allovir: Consultancy; AbbVie: Consultancy, Honoraria; DSMB: Other; Allogene: Research Funding; Equillium: Consultancy, Honoraria; Miltenyi Biotec: Honoraria. Riedell:Nkarta: Research Funding; Genmab: Consultancy; Pharmacyclics: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CVS Caremark: Consultancy; Sana Biotechnology: Consultancy; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; Calibr: Research Funding; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Research Funding; Xencor: Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Roche: Research Funding; CRISPR Therapeutics: Research Funding; Janssen: Consultancy. Shpall:Adaptimmune: Membership on an entity's Board of Directors or advisory committees; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Affimed: Other: License agreement; Takeda: Other: License agreement; Axio: Membership on an entity's Board of Directors or advisory committees; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; Syena: Other: License agreement. Sorror:JAZZ pharmaceuticals: Consultancy; BlueNote and Massachusetts General Hospital: Research Funding. Turtle:CJT has the right to receive payment from Fred Hutch as an inventor on patents related to CAR T-cell therapy: Other: Patents; Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics: Other: Stock options; Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics/BMS, Nektar Therapeutics: Research Funding; Nektar Therapeutics, Century Therapeutics, Legend Biotech, Allogene, Sobi, Syncopation Life Sciences, Prescient Therapeutics: Other: Ad hoc advisory boards/consulting (last 12 months); Kyverna: Other: DSMB Member. Pasquini:Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Kite, a Gilead Company: Honoraria, Research Funding; Janssen: Research Funding; Kite Brazil: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal